1. What is a thermal power system?
A thermal power system is a system that converts energy from one form to another. More specifically, these systems convert heat into mechanical power. This mechanical power can subsequently be used directly, for example to power a car or aircraft, or converted into electricity, as is the case in large-scale power stations. Conventionally, the heat used to drive these systems has been derived from fossil fuels, but this heat could equally come from renewable sources such as the sun, deep under the ground, biomass or industrial waste heat.
The key building blocks of most thermal power systems are the following four processes:
- compression is the process of increasing the pressure of a gas or liquid through a reduction in volume. This process requires work to be done on the system, and is thus associated with the addition of energy to the system. A simple example is to consider an empty plastic bottle that has the lid screwed on. At rest, there is a certain amount of air trapped within the bottle. If you squeeze the sides of this bottle the space inside it will reduce and the air molecules will be pushed closer together. This causes an increase in the air pressure. You would also notice that in the process of squeezing the bottle your muscles are being made to work, and thus you are adding energy to the air trapped within the bottle.
- heating is the process of increasing the temperature of a gas or liquid through the addition of energy in the form of heat. This heat is supplied by the heat source, be that fossil fuels or a renewable resource.
- expansion is the opposite of compression and is the process of reducing the pressure of a gas or liquid through an increase in volume. Whilst compression is associated with doing work on the system, expansion is the opposite and is associated with the system doing work. Thus, energy is transferred away from the system, and it is this energy that can be harnessed as mechanical power, or converted into electricity.
- cooling is the process of reducing the temperature of a gas or liquid through cooling it. This is the opposite of heating, in that this is associated with the transfer of heat outside of the system to the surroundings.
In a thermal power system, these four processes are completed sequentially, and are coupled together to form a closed-loop system, as shown below.
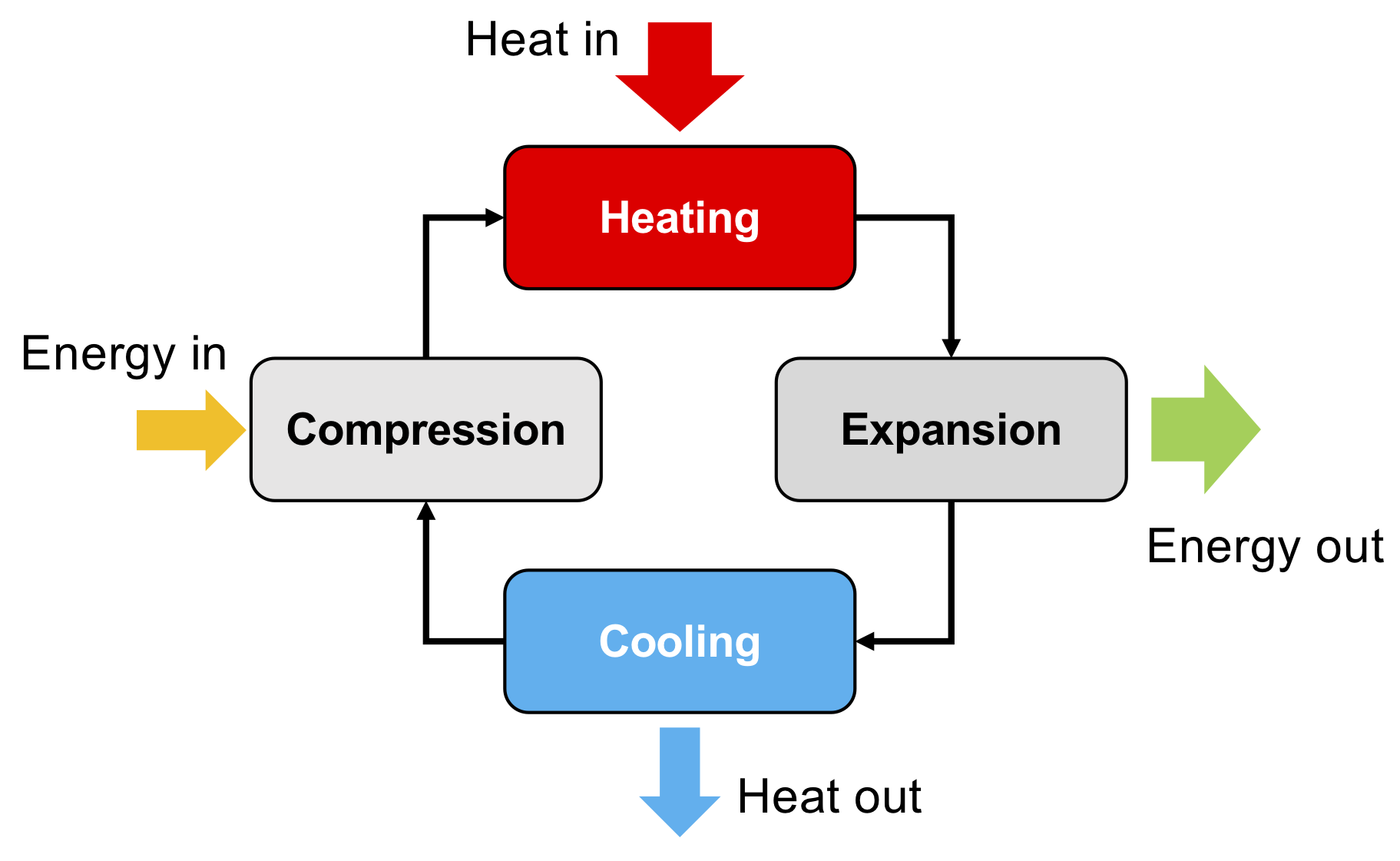
Diagram of the operating principle of a basic thermal power system.
Within such a system, the total energy added to the system is equal to the total energy that is taken away from the system. In other words, the combined total of the energy added during the compression process and the heat added during the heating process is equal to the combined total of the energy released during the expansion process and the heat taken away during the cooling process.
The net result of the cycle is the generation of useful work since the work required for the compression process is less than the energy released during the expansion process. Essentially, the amount of energy required for compression, and the amount of energy released during expansion, increase as the temperature of the fluid undergoing the compression or expansion process increases. Therefore, by having an expansion process that occurs at high temperature, and a compression process that occurs at a low temperature, it means the energy required for the low-temperature compression is lower than the energy released during the high-temperature expansion. Thus, from this cycle the net result is the generation of useful power from the system.
It is worth noting that the cooling process is only required to complete the cycle and return the cycle back to its starting point. In some thermal power systems that do not operate in a closed loop, this process is removed.
When talking about thermal power systems, you will often encounter the concept of the cycle efficiency. This is defined as the ratio of the amount of useful energy produced by the system to the amount of heat supplied to the system. By way of a simple example, consider a thermal power system that is driven by 100 units of energy (heat). Within this system, 10 units of energy are required for the compression process, whilst the expansion process generates 35 units units of energy. What is the cycle efficiency?
Firstly, the net energy produced by the cycle is equal to 25 units of energy (expansion work minus compression work = 35 - 10).
The cycle efficiency is then 25% (net energy produced divided by heat input = 25/100).
The amount of heat released to the surrounding during the cooling process is 75 (compression + heat input - expansion = 10 + 100 - 35).